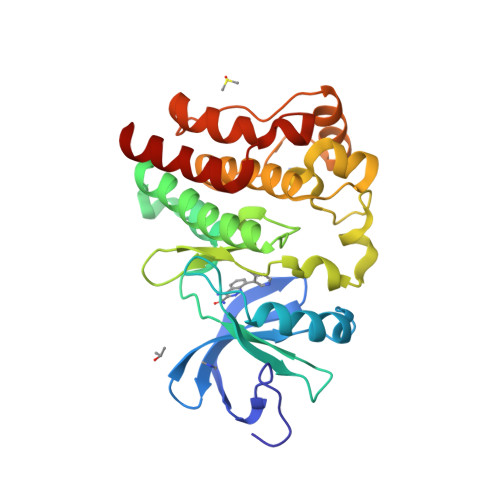
Fragment-Based Discovery of a Small Molecule Inhibitor of Bruton's Tyrosine Kinase.
Smith, C.R., Dougan, D.R., Komandla, M., Kanouni, T., Knight, B., Lawson, J.D., Sabat, M., Taylor, E.R., Vu, P., Wyrick, C.(2015) J Med Chem 58: 5437-5444
- PubMed: 26087137
- DOI: https://doi.org/10.1021/acs.jmedchem.5b00734
- Primary Citation of Related Structures:
4Z3V, 4ZLY, 4ZLZ - PubMed Abstract:
The discovery and optimization of a series of 4-aminocinnoline-3-carboxamide inhibitors of Bruton's tyrosine kinase are reported. A fragment-based screening approach incorporating X-ray co-crystallography was used to identify a cinnoline fragment and characterize its binding mode in the ATP binding site of Btk. Optimization of the fragment hit resulted in the identification of a lead compound which reduced paw swelling in a dose- and exposure-dependent fashion in a rat model of collagen-induced arthritis.
- Takeda California, 10410 Science Center Drive, San Diego, California 92121, United States.
Organizational Affiliation: